Aiming for Next Level Innovation
Theon Lifesciences Ltd. is among India’s leading sterile injectable manufacturers, built to meet the standards of the world’s most regulated markets. Operating from a 4,55,000 sq. ft. USFDA, WHO-GMP, and ISO-certified facility, we ensure precision, scalability, and compliance at every step, from high-speed automated lines to NABL-accredited labs and intelligent data systems.
With a strong foothold in pharma formulations, We are rapidly growing as a trusted global partner, delivering affordable and innovative injectable therapies across Europe, the USA, Latin America, South Africa, and Australia.
Our mission: To make world-class injectable solutions accessible through quality, efficiency, and trust.

Our Production Capabilities
Our dedicated production lines for sterile injectable includes:

Liquid Injectables

Ampoules

Lyophilized Injectables

Ophthalmic Drops

Pre-filled Syringes
About Theon Lifesciences
With a dominant focus on sterile formulations, we deliver world-class quality injectables and operate under cGMP-certified protocols, combining cutting-edge infrastructure, stringent quality systems, and regulatory excellence for patients, professionals, and partners across the world. Our facility spans 10.46 acres with a 4,55,000 sq. ft. injection block, designed in compliance with EU GMP, USFDA, TGA Aus, and ANVISA guidelines, ensuring smooth operations with minimal human intervention.
Theon in Brief
Technology Transfer
We Undertake The Technology Transfer For Liquid vials, Ampoules, PFS and Ophthalmic Generic Formulations.
Media Fills Readiness
All lines are qualified and ready for media fill in this month of May 2025.
Ampoule Line
Automatic Ampoule loading, washing machine, tunnel filling and sealing machine is qualified along with terminal sterilizer, labelling machine and Blister machine and complete Ampoule line set up is ready for operations.
Ophthalmic Line
Ophthalmic Filling and sealing Machine ROMACO (3 piece) is qualified and ready for operations
Aseptic Process
Aseptic Process Simulation Activity is Scheduled in May 2025 and is under process.
Manufacturing Lines
Our all areas of four lines: vial, ampoules, PFS, and Ophthalmic are Qualified for continuous sterile manufacturing.
Complete Vial Line
Complete vial line starting from loading of vials, washing, 04 tunnel, filling and sealing is qualified along with Automatic Loading and un-loading system for Lyophilizer and lyophilizer is qualified and ready operations.
Pre-Filling Syringe Line
PFS Filling Machine is Under Qualification.
Manufacturing Vessel System
Manufacturing Vessel System Compounding and Holding 08 vessels are qualified for all four lines and ready for operations.
Media Fill Activities
Media Fill activities will Be Completed in June 2025 Before Starting the Commercial Production.
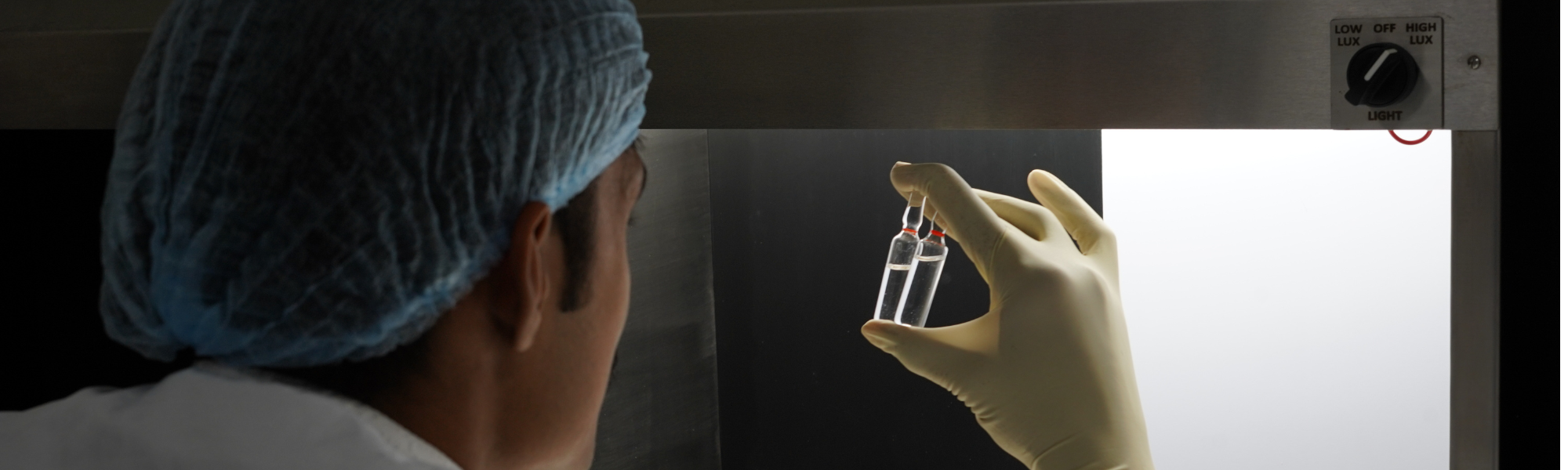
Our Core Strengths in Global Manufacturing
Advanced R&D Centre
We are a dedicated hub for formulation development and innovative sterile injectables, focused on driving global market readiness. Our expertise spans complex generics, novel delivery systems, and seamless technology transfer support.
Tech Excellence and Expertise
We are specialized in manufacturing liquid vials, ampoules, lyophilized injectables, pre-filled syringes, and ophthalmic preparations. Our advanced technology transfer capabilities ensure regulatory compliance, and faster market entry for complex generics and novel formulations.
High-Production Capacity
With a world-class infrastructure, we can produce high-volume, and reliable production. We can adjust to market demands because of our automation and process optimization, guaranteeing a reliable supply chain for both developing and heavily regulated international markets.
Environment Friendly Operations
We are committed to build an eco-conscious manufacturing facility as our units are equipped with 100 KLD ETP and 50 KLD STP for wastewater treatment. From waste minimization to responsible sourcing of raw materials, every step reflects our dedication to sustainability.
Why Choose Theon Lifesciences?
Global Compliance
Facility validated for stringent international regulatory requirements.
Cutting-Edge Technology
From robotic PFS filling to advanced compounding and vessel systems.

Customer-Centric
Focused on creating value for global partners through reliability and innovation.
Quality First
21 CFR part 11 compliant systems with real-time monitoring and traceability.
High-Capacity Manufacturing
Automated lines capable of handling millions of units annually.
Why Choose Theon Lifesciences?

Global Compliance
Facility validated for stringent international regulatory requirements.
Cutting-Edge Technology
From robotic PFS filling to advanced compounding and vessel systems.
High-Capacity Manufacturing
Automated lines capable of handling millions of units annually.
Quality First
21 CFR part 11 compliant systems with real-time monitoring and traceability.
Customer-Centric
Focused on creating value for global partners through reliability and innovation.
Building a Safer, Greener Tomorrow
Water Management
Rainwater collection and water reuse
Green Operations
Our objective is simple: to develop life-saving medications while also preserving the environment.

Certifications and Compliance
Our Globally Recognized Quality Standards and Certifications



Global Trust, Built on Proven Scale
Measurable Results Delivered with our Manufacturing Excellence
Trusted by Leading Pharma Brands
What our pharmaceutical partners say about us or Insights from Our Global Network
Dr. Sarah Johnson
Global Pharma Corp

Michael Chen
MedTech Solutions

BioHealth International
Global Pharma Corp
News & Events
Our News & Events section highlights the recognitions, achievements, and global engagements that shape our journey.
News & BLogs
Stay updated with the latest news, insights, and blogs featuring industry trends, expert tips, and updates to keep you informed and inspired
Ready to Partner with Us?
Let’s discuss how Theon Lifesciences can support your pharmaceutical manufacturing needs with ethical innovation and global compliance.






